Abstract
Corresponding Author:
Muhammad Umair Mushtaq, MD
Assistant Professor of Medicine
Division of Hematologic Malignancies and Cellular Therapeutics
University of Kansas Medical Center
2330 Shawnee Mission Parkway, Suite 210, MS 5003, Westwood, KS 66205
Email: mmushtaq@kumc.edu
Background:
Anti CD19 chimeric antigen receptor (CAR) T cell therapy is being utilized for the treatment of patients with refractory and relapsing hematologic malignancies with propitious results. It has resulted in a significant increase in overall survival and reduced relapse rate. However, a multitude of cardiac complications has been observed with this therapy. This systematic review aims at cardiovascular complications of CD 19 CAR-T cell therapy.
Methodology:
Following the preferred reporting items for systemic reviews and Meta-analysis (PRISMA) guidelines, a comprehensive literature search was performed on three databases (PubMed, Cochrane Register of Controlled Trials, and Clinicaltrials.gov) using MeSH terms and keywords for "cardiovascular complications" AND "CAR-T Cell therapy" from the date of inception to May 16, 2022. Our search produced 354 results, and duplicates were removed. After excluding irrelevant and review articles during primary and secondary screening, ten studies were included which mentioned cardiovascular side effects of CAR-T cell therapy.
Results:
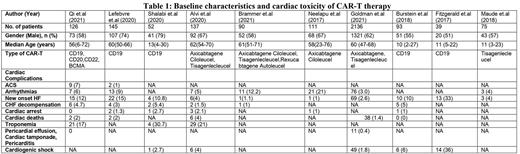
A total of 3004 patients from ten studies were included for this systematic review. The median age of patients was 57 (2-72) years, and 62% were male. (Table 1) All included studies used CD19 CAR-T cell therapy except Qi et al. which used CD20 and CD22 in addition to CD19. Eight studies were retrospective, and two were clinical trials. As per the available data, Troponemia was the most commonly reported cardiac side effect of CART therapy with an incidence of 18.3% (n=54), followed by arrhythmias at 4.9% (n=138), new onset of congestive heart failure 4.8% (n=144), and acute coronary syndrome (ACS) 4.1% (n=11). CHF decompensation, cardiogenic shock, venous thromboembolism, and myocarditis was observed in 3.2% (n=20), 3.1% (n=76), 1.5%(n=35), 0.8% (n=4) of the patients, respectively. Cardiac arrest due to CAR-T cell therapy occurred in 1.5% (n=8) of patients, while death due to cardiac causes occurred in 1.9% (n=48) of the patients.
Conclusion:
The overall rate of cardiotoxicity associated with CAR-T cell therapy is low and further studies are need of the hour to consolidate these findings.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal